Opioid overdose deaths in England have risen by 32% in 2013, as reported by the Office for National Statistics. Urgent action is needed to ensure that overdose deaths return to the previous downward trend.
This rise in deaths occurred only in England – Wales, Northern Ireland and Scotland already have a national take home naloxone programmes which have contributed to deaths remaining static in Wales and falling in Scotland.
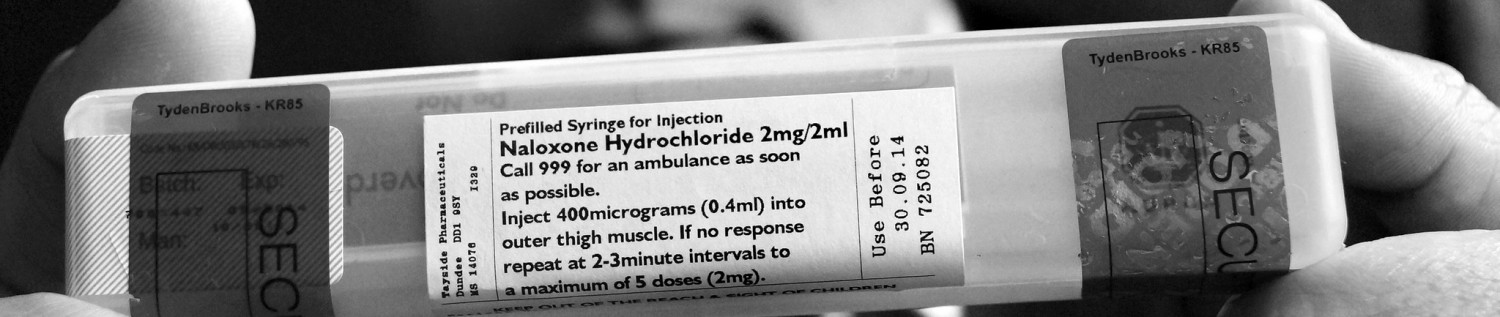
Naloxone is already licensed for use in the treatment of suspected acute opioid overdose or intoxication and it is being used effectively in a number of local authority areas in England, but there are many where plans are unclear or undeveloped meaning there is a marked variation in access across the country.
In May 2012 the Advisory Council on the Misuse of Drugs (ACMD) reviewed the availability of naloxone in the UK and recommended to the government that it should be more widely available to tackle the high numbers of fatal opioid overdoses. ACMD advised that the Government should ease the restrictions on who can be supplied with naloxone and should investigate how people supplied with naloxone can be suitably trained to administer it in an emergency and respond to overdoses.
The government have now responded to this recommendation and have said that new regulations are being prepared by the Medicines and Healthcare Products Regulatory Agency (MHRA) which will come into effect in October 2015.
We call on:
- The MHRA to publish their draft regulations on naloxone now.
- The Department of Health to implement a national programme to ensure that take-home naloxone is made available to those vulnerable to opioid overdose, as well as those likely to be present, such as family members and carers.
- Public Health England to make every effort to encourage local authority areas to make the availability of naloxone and accompanying training a priority, and to monitor and report on activity.
- For public health teams in local authorities to commission services in line with the recommendations from the World Health Organisation (WHO), the UN Commission on Narcotic Drugs and the ACMD on the community management of overdose. For example, the World Health Organisation (WHO) have specifically recommended that people likely to witness an opioid overdose should have access to naloxone and be instructed in its administration to enable them to use it for the emergency management of suspected opioid overdose.
Signatories
- Action on Addiction
- Addaction
- Adfam
- Blenheim CDP
- Bridge
- Brighton Oasis Project
- Bristol Drug Project
- B3 – Brent Service User Council
- Compass
- CRANSTOUN
- Crawley Exact
- CRI
- DrugScope
- EDP
- Herefordshire Family Drug Support
- HIT
- Homeless Link
- International Doctors for Healthier Drug Policies (IDHDP)
- International Drug Policy Consortium
- Kenward Trust
- National Needle Exchange Forum (NNEF)
- Nottingham CDP
- Release
- Service User Strategy 4 Self Empowerment & Development (SUSSED (Bedford))
- SHP
- Skills Consortium
- Stepping Stones (Luton)
- Substance Misuse Management in General Practice (SMMGP)
- Swanswell
- Telford Aftercare Team (TACT)
- UK Recovery Walk
- United Kingdom Harm Reduction Alliance (UKHRA)
- Wolverhampton Drug Service User Involvement Team
If you would like to be a signatory to this call for action please let us know
Don’t like the protocol in the photo no mention respiratory assist. Medical consensus, ERC; ILCOR; AHA ad fin item. Simple logic RESPIRATORY EMERGENCY
https://jgarythompson.wordpress.com/2015/08/11/agnotology/
LikeLike
ALSO be aware risk of naloxone in opiate tolerant users of opiates for pain relief, especially those with cardiac conditions: https://www.england.nhs.uk/patientsafety/wp-content/uploads/sites/32/2015/10/psa-naloxone-stage2.pdf
LikeLike
The problem is Naloxone without rescue breathing is gross incompetence. Beyond grey medical literature live human study in Ontario, chest compressions only for respiratory emergency Can. J. Public Health 2013;104(3):e200-4
‘Development and implementation of an opioid overdose prevention and response program in Toronto, Ontario.’ http://static.smallworldlabs.com/hsf/user_content/files/000/000/169/355cc02324a166bb8abf31174c141f69-cjph-20131043200-4.pdf
Emergency Medicine News Dec. 2015
http://journals.lww.com/em-news/Fulltext/2015/12000/Letter__Flaws_in_Toronto_s_Opioid_Overdose.14.aspx
Was also published in the 2015 AHA & ILCOR CPR guidelines about this life threatening intervention. Read all comments under this deputation Toronto Board of Health https://youtu.be/QhsDjmI9H9c
Disclosure I am not an MD.
LikeLike
If someone’s not breathing or becoming cyanosed it would the lesser of all the worst outcomes to administer Naloxone. A simple gouch does not require one to be running around with needles and being a “Super hero” Thus, the importance of good training and information.
LikeLike
Naloxone has come so far here in England but there is still resistance in some areas where statistically there is evident need for a program. After speaking to a number of individuals both in those areas and in other areas where uptake has not been great I have found that there is not only a resistance to Naloxone but, more prominently, there is a resistance to the fact it is in an injectable form. A headline in the Vancouver Sun revealed that despite there being an epidemic of Fentanyl deaths in Vancouver and surrounding areas, students at University of British Columbia were refusing to take free kits handed out in an attempt to address the epidemic. I delved into this a bit deeper and found that it was quite a huge barrier. Healthcare professionals are beginning to pull back on administering injectable medicines due to the high prevalence of Hepatitis C. I have personal experience of that where I was getting my bloods done and the nurse was struggling, I told her I was a recovering individual and she immediately put the needle down, and quite abruptly demanded why I hadn’t told her earlier and advised me that a specialist would be there to tend to me soon. I was then left waiting for a further 2.5 hours until a specialist was available. Carers and similar are concerned around carrying injectable medications around with them and primarily parents are resistant to having injectable medications in the family home.
There might be a need to revisit the initial drive and introduce a non injectable form of Naloxone alongside the already available injectable. If people are refusing to take up kits due to the injectable status, then it is definitely something that needs to be addressed. A choice needs to be made available. Needs to be a topic at next meeting. And, it’s not going to be an easy pitch either. There have been a number of rejections from FDA and European Medicines. Although I believe there has been an approval by FDA recently there has also been a refusal to license intranasal as an orphan drug this week at the European Medicines Agency here in London. Perhaps there is a good place to start.
LikeLike